Biological causes of osteoporosis
In adults, the daily removal of small amounts of bone mineral, a process called resorption, is balanced by an equal deposition of new mineral in order to maintain bone strength. When this balance tips toward excessive resorption, bones weaken and over time can become brittle and prone to fracture (osteoporosis).
This continual resorption and re-deposition of bone mineral, or bone remodelling, is intimately tied to the pathophysiology of osteoporosis. Understanding how bone remodelling is regulated is the key to the effective prevention and treatment of osteoporosis.
Bones have evolved to be light yet strong. These properties are conferred to a large degree by architecture and geometry [2]Martin, R.M. and P.H. Correa, Bone quality and osteoporosis therapy. Arq Bras Endocrinol Metabol, 2010. 54(2): p. 186-99.
. The long bones are tubular in shape, with a strong outer shell, or cortical layer, surrounding a spongier core called trabecular bone [3]Parfitt, A.M., Chapter 15 - Skeletal Heterogeneity and the Purposes of Bone Remodeling: Implications for the Understanding of Osteoporosis, in Osteoporosis (Second Edition), R. Marcus, D. Feldman, and J. Kelsey, Editors. 2001, Academic Press: San Diego. p. 433-447.
. The combination makes these bones strong and light, but flexible enough to absorb the stress – from high impact exercises – without breaking. The vertebrae are similarly constructed, with a thick cortical layer surrounding sheets of trabecular bone. As a unit, each vertebra can compress when temporarily loaded and then return to their original size.
The influence of bone geometry on bone strength [2]Martin, R.M. and P.H. Correa, Bone quality and osteoporosis therapy. Arq Bras Endocrinol Metabol, 2010. 54(2): p. 186-99.
.
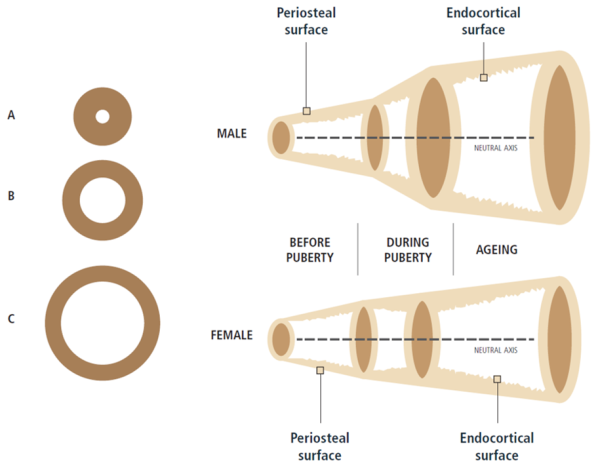
LEFT: For the same areal BMD, bone C has progressively greater bending strength and axial strength than bone B and bone A because the mass of bone C is distributed further away from the centre [1]Bouxsein, M.L., Determinants of skeletal fragility. Best Pract Res Clin Rheumatol, 2005. 19(6): p. 897-911.
.
RIGHT: Sex and ageing differences in periosteal apposition and endocortical resorption in tubular bones [4]Seeman, E., Bone quality: the material and structural basis of bone strength. J Bone Miner Metab, 2008. 26(1): p. 1-8.
.
However, a skeleton is alive and must be able to grow, heal, and respond to its environment. This is where bone remodelling plays a crucial role. However, as we age, daily remodelling leads to a gradual resorption of the minerals on the inside of the cortical layer and in the bone cavity itself leads to an inexorable loss of trabecular bone and a widening of the bone cavity. This is partly compensated for by the gradual addition of extra layers of mineral to the outside of the cortical layer [5]Seeman, E., From density to structure: growing up and growing old on the surfaces of bone. J Bone Miner Res, 1997. 12(4): p. 509-21
.
Continual remodelling, and its effect on bone microarchitecture have a huge impact on the pathophysiology of osteoporosis. For example, young adults with wider femurs might be at higher risk for hip fractures late in life because, on average, wider bones tend to have thinner cortical layers. The thinner this layer is, the more susceptible it will be to resorption later in life [6]Seeman, E. and P.D. Delmas, Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med, 2006. 354(21): p. 2250-61
.
Figure taken from Ferrari & Roux, 2019 [7]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
.
The cellular connection
The balance between bone resorption and bone deposition is determined by the activities of two principle cell types, osteoclasts and osteoblasts, which are from two different origins. Osteoclasts are endowed with highly active ion channels in the cell membrane that pump protons into the extracellular space, thus lowering the pH in their own microenvironment [8]Blair, H.C., et al., Osteoclastic bone resorption by a polarized vacuolar proton pump. Science, 1989. 245(4920): p. 855-7.
. This drop in pH dissolves the bone mineral. They also produce in this microenvironment proteolytic enzymes, among them cathepsin K, which dissolve bone matrix. Osteoblasts, through a yet poorly characterized mechanism, lay down new bone mineral. The balance between the activities of these two cell types governs whether bone is made, maintained, or lost. The activities of these cells are also intimately intertwined.
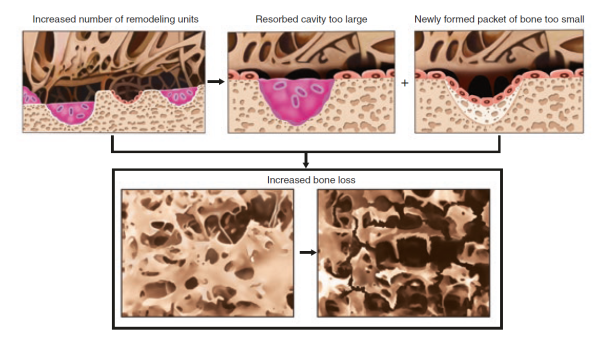
In a typical bone remodelling cycle, osteoclasts are activated first, leading to bone resorption (see bone biology – bone remodelling). Then, after a brief “reversal” phase, during which the resorption “pit” is occupied by osteoblasts precursors, bone formation begins as progressive waves of osteoblasts form and lay down fresh bone matrix [9]Orwoll, E.S., Toward an expanded understanding of the role of the periosteum in skeletal health. J Bone Miner Res, 2003. 18(6): p. 949-54.
. Because the bone formation phase typically takes much longer than the resorption phase, any increase in remodelling activity tends to result in a net loss of bone. At various stages throughout this process, the precursors, osteoclasts, and osteoblasts communicate with each other through the release of various “signalling” molecules [6]Seeman, E. and P.D. Delmas, Bone quality--the material and structural basis of bone strength and fragility. N Engl J Med, 2006. 354(21): p. 2250-61
[10]Raisz, L.G., Pathogenesis of osteoporosis: concepts, conflicts, and prospects. J Clin Invest, 2005. 115(12): p. 3318-25.
. How these signalling molecules and various other endogenous (such as hormones) or external (such as diet and exercise) factors influence the cells involved in bone physiology is a topic of intense research activity.
Factors influencing osteoclasts and osteoblasts
Hormones are possibly the most crucial modulators of bone formation. It is well established that oestrogen [11]Lindsay, R., Prevention and treatment of osteoporosis. Lancet, 1993. 341(8848): p. 801-5.
. parathyroid hormone [12]Lips, P., Vitamin D physiology. Prog Biophys Mol Biol, 2006. 92(1): p. 4-8
. and to a lesser extent testosterone directly or indirectly via the conversion into oestrogen [13]Seeman, E., The structural basis of bone fragility in men. Bone, 1999. 25(1): p. 143-7.
[14]Van Pottelbergh, I., et al., Perturbed sex steroid status in men with idiopathic osteoporosis and their sons. J Clin Endocrinol Metab, 2004. 89(10): p. 4949-53.
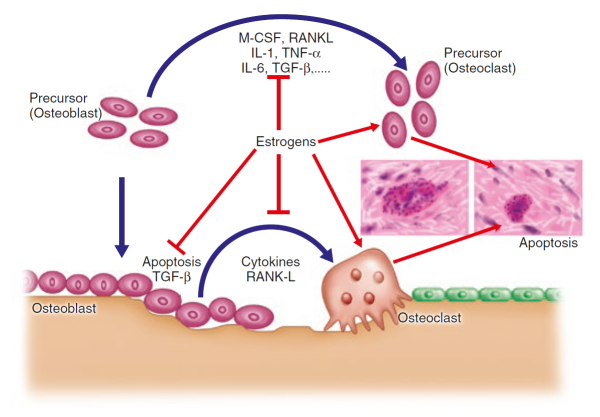
. are essential for optimal bone development and maintenance. Of these, oestrogen is now believed to have the most direct effect on bone cells, interacting with specific proteins, or receptors, on the surface of osteoblasts and osteoclasts [15]Zallone, A., Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann N Y Acad Sci, 2006. 1068: p. 173-9
.
This interaction sets off a complex chain of events within the cells, increasing osteoblast activity while at the same time interfering with osteoblast-osteoclast communication – one of the ironies of bone remodelling is that the osteoblasts release factors that stimulate osteoclasts and drive bone resorption, as we shall see below.
Oestrogen effects are mediated through one specific type of cell surface receptor called the oestrogen receptor alpha (ERα), which binds and transports the hormone into the nucleus of the cell where the receptor-hormone complex acts as a switch to turn on specific genes. ERα receptors are found on the surface of osteoblasts, as is oestrogen receptor-related receptor alpha (ERRα), which may play an auxiliary role in regulating bone cells [16]Bonnelye, E. and J.E. Aubin, Estrogen receptor-related receptor alpha: a mediator of estrogen response in bone. J Clin Endocrinol Metab, 2005. 90(5): p. 3115-21.
. Recent studies also suggest that sex hormone binding globulin (SHBG), which facilitates entry of oestrogen into cells, may also play a supportive role [17]Goderie-Plomp, H.W., et al., Endogenous sex hormones, sex hormone-binding globulin, and the risk of incident vertebral fractures in elderly men and women: the Rotterdam Study. J Clin Endocrinol Metab, 2004. 89(7): p. 3261-9.
.
Oestrogen, of course, is made and secreted into the bloodstream some distance from bone and it also has profound effects on other tissues, such as the uterus and breast. But there are other, locally produced signalling molecules that have profound effects on bone physiology.
Figure taken from Ferrari & Roux, 2019 [7]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
.
Prostaglandins, particularly prostaglandin E2 (PGE2), stimulate both resorption and formation of bone [18]Pilbeam, C.C., J.R. Harrison, and L.G. Raisz, Chapter 54 - Prostaglandins and Bone Metabolism, in Principles of Bone Biology (Second Edition), J.P. Bilezikian, L.G. Raisz, and G.A. Rodan, Editors. 2002, Academic Press: San Diego. p. 979-994
. PGE2 is a lipid that is formed in various bone cells from a precursor called arachidonic acid. The first step on PGE2 synthesis is carried out by an enzyme called cyclooxygenase 2 (COX2) and inhibitors of this enzyme can prevent bone formation in response to mechanical stress in animals [19]Forwood, M.R., Inducible cyclo-oxygenase (COX-2) mediates the induction of bone formation by mechanical loading in vivo. J Bone Miner Res, 1996. 11(11): p. 1688-93.
. PGE2 may be required for exercise-induced bone formation.
There is evidence that fracture risk is increased in people taking non-steroidal anti-inflammatory drugs that inhibit COX-2 [20]Carbone, L.D., et al., Association between bone mineral density and the use of nonsteroidal anti-inflammatory drugs and aspirin: impact of cyclooxygenase selectivity. J Bone Miner Res, 2003. 18(10): p. 1795-802
may also increase. Another set of lipid molecules that appear to regulate bone remodelling are the leukotrienes. Also derived from arachidonic acid, these have been found to reduce bone density in mice [21]Traianedes, K., et al., 5-Lipoxygenase metabolites inhibit bone formation in vitro. Endocrinology, 1998. 139(7): p. 3178-84.
.
How any of these hormones impact bone remodelling depends on how they alter osteoclasts and/or osteoblasts activity. Specific cell surface receptors help to transmit signals from outside bone cells into the cell nucleus, where different genes that regulate cell activity can be switched on or off. These include receptors for bone morphogenetic proteins (BMPs) a family of proteins which are potent inducers of bone formation.
BMP receptors have been found on the surface of osteoblasts precursor cells [22]Mbalaviele, G., et al., Beta-catenin and BMP-2 synergize to promote osteoblast differentiation and new bone formation. J Cell Biochem, 2005. 94(2): p. 403-18.
. Another cell surface receptor called the low-density lipoprotein (LDL)-related protein 5 receptor (LRP5), a Wnt receptor, may also be important for bone formation because loss of LRP5 in animals leads to severe osteoporosis [23]Gong, Y., et al., LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell, 2001. 107(4): p. 513-23.
. BMP receptors and LRP5 may cooperate to stimulate osteoblasts into action, though exactly how this might occur has not been clarified.
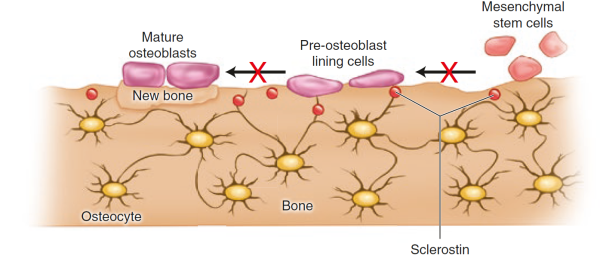
Sclerostin, product of the SOST gene and expressed by the osteocytes, binds to LRP5/6 receptor on osteoblasts and inhibits the Wnt signalling, leading to a decrease in bone formation [24]Bonewald, L.F., The amazing osteocyte. J Bone Miner Res, 2011. 26(2): p. 229-38.
[25]Li, X., et al., Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem, 2005. 280(20): p. 19883-7.
. Parathyroid hormone (PTH) and mechanical loading decrease the secretion of sclerostin by the oesteocytes [26]Bellido, T., et al., Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis.Endocrinology, 2005. 146(11): p. 4577-83.
[27]Robling, A.G., et al., Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem, 2008. 283(9): p. 5866-75.
. An antibody against sclerostin has been developed as a potential drug with potent properties on bone strength. Read more on anabolics as treatments.
Figure taken from Ferrari & Roux, 2019 [7]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
.
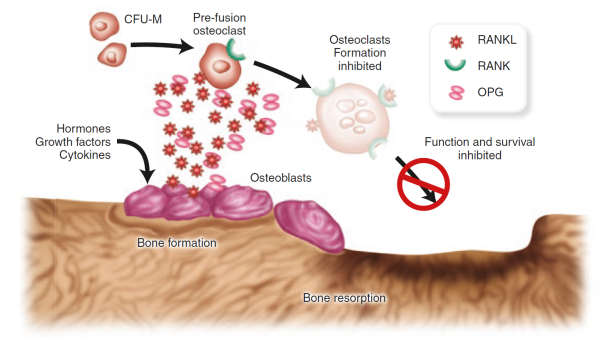
A cell surface receptor called RANK (for receptor activator of NFκB) prods osteoclasts precursor cells to develop into fully differentiated osteoclasts when RANK is activated by its cognate partner RANK ligand (RANKL) [28]Yasuda, H., et al., Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A, 1998. 95(7): p. 3597-602.
[29]Lacey, D.L., et al., Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell, 1998. 93(2): p. 165-76.
.
RANKL, in fact, is produced by osteoblasts and is one of perhaps many signalling molecules that facilitate cross-talk between the osteoblasts and osteoclasts and help coordinate bone remodelling [30]Theill, L.E., W.J. Boyle, and J.M. Penninger, RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol, 2002. 20: p. 795-823.
. Osteoprotegerin, another protein released by osteoblasts [31]Suda, T., et al., Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocr Rev, 1999. 20(3): p. 345-57.
, can also bind to RANKL, acting as a decoy to prevent RANK and RANKL from coming in contact. The balance of RANKL/osteoprotegerin may be crucial in osteoporosis. In fact, animal studies showed that increased production of osteoprotegerin leads to an increase in bone mass, while loss of the protein leads to osteoporosis and increased fractures [32]Bucay, N., et al., osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev, 1998. 12(9): p. 1260-8.
. Inhibitors of RANKL have also shown promise as potential treatment for osteoporosis in humans.
Figure taken from Ferrari & Roux, 2019 [7]Pocket Reference to Osteoporosis, S. Ferrari, Roux, C., Editor 2019, Springer International Publishing.
.
A second, complementary cell signalling system that helps drive formation and activation of osteoclasts was also uncovered within the last few years. In the absence of DNAX-activating protein 12 (DAP12) and Fc Receptor common γ chain (FcRγ), two cell surface receptors, mice develop severe osteoporosis – the exact opposite of osteoporosis – characterized by a dramatic increase in bone density [33]Mocsai, A., et al., The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci U S A, 2004. 101(16): p. 6158-63.
[34]Koga, T., et al., Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature, 2004. 428(6984): p. 758-63.
. These two cell surface receptors interact with a group of proteins in the cell called ITAM (immunoreceptor tyrosine-based activation motif) adaptor proteins to cause an increase in intracellular calcium.
Studies suggest that the RANK/RANKL and the ITAM-mediated pathways cooperated to induce full osteoclasts activity. These two pathways may converge to activate a protein called the nuclear factor of activated T cells (NFAT) c1. NFATc1 serves as a master switch for bone resorption because it turns on the genes that osteoclasts precursor cells need to become fully active osteoclasts [35]Takayanagi, H., Mechanistic insight into osteoclast differentiation in osteoimmunology. J Mol Med (Berl), 2005. 83(3): p. 170-9.
.
The role of genetics and environmental factors
Subtle differences in the genetic code might explain why one person’s osteoblasts or osteoclasts are more active or responsive to their environment, and it might also lead to the discovery of unknown regulatory mechanisms. Environmental factors can also have an enormous impact on bone physiology. See risk factors for more information.